The 2026 flu season is shaping up to look very different. With the transition from QIV (Quadrivalent Influenza Vaccine) to TIV (Trivalent Influenza Vaccine), there will be fewer vaccine options available. Manufacturers have already indicated that their production capacity is limited, and the likelihood of shortages has significantly increased.
With only so much stock available, it has never been more important to place your 2026 flu vaccine order early. Acting now ensures you:
• Lock in your supply ahead of the rush.
• Access early bird pricing — maximise value while securing stock.
• Plan with confidence knowing your patients will be protected
Products Available for 2026 Influenza Season Pre-Order
Flu Vaccines: Transition from Quadrivalent to Trivalent
What this means for you:
• Reduced choice: With less variety in flu vaccines, securing your preferred option early is critical.
• Higher risk of supply gaps: Once stock is allocated, it may not be replenished.
• Increased demand: Every provider is competing for limited supply.
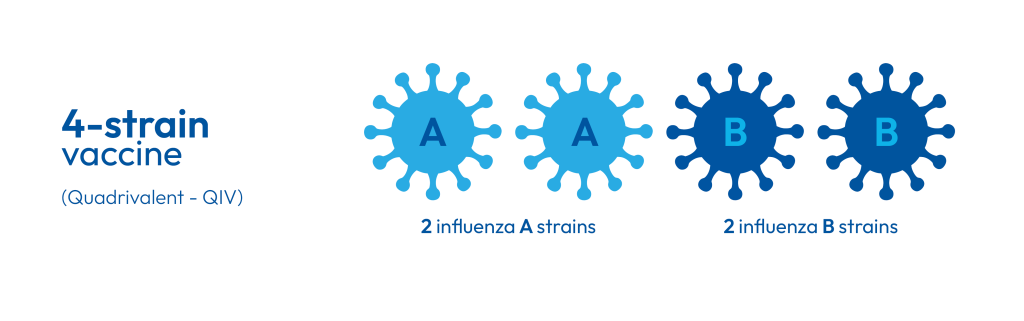
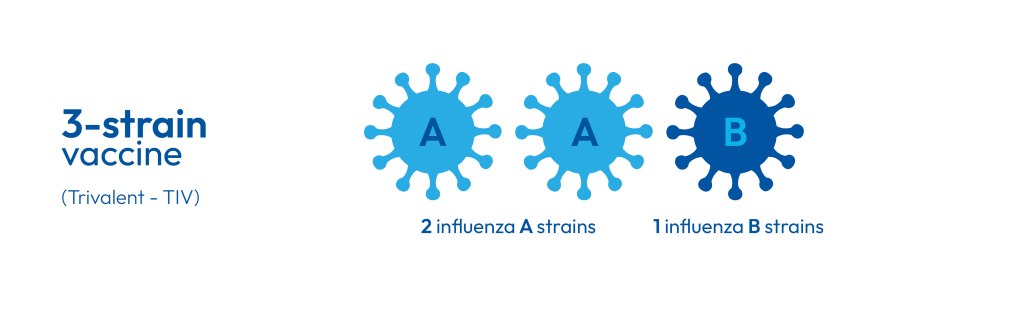


The B/Yamagata influenza B strain has not been detected since the COVID-19 pandemic. As a result, the World Health Organization (WHO) now recommends removing it from seasonal influenza vaccines (1). From the 2026 Winter Season, manufacturers will follow this guidance by transitioning from quadrivalent (QIV) to trivalent (TIV) formulations (2). This ensures vaccines remain effective while avoiding unnecessary strain coverage. This change mirrors the Northern Hemisphere, which began its transition during the 2024–2025 season (3).
References: 1. World Health Organisation. Recommended composition of influenza virus vaccines for use in the 2025-2026 northern hemisphere influenza season. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2025-2026-nh-influenza-season (Accessed November 2025); 2. ATAGI. Statement on the transition from quadrivalent to trivalent seasonal influenza vaccines in Australia. https://www.teammed.com.au/team/wp-content/uploads/atagi-statement-on-the-transition-from-quadrivalent-to-trivalent-seasonal-influenza-vaccines-in-australia.pdf (Accessed July 2025); 3. FDA. Use of trivalent influenza vaccines for the 2024-2025 U.S influenza season. https://www.fda.gov/vaccines-blood-biologics/lot-release/use-trivalent-influenza-vaccines-2024-2025-us-influenza-season (Accessed July 2025).
Don't Delay - Order Today!
Why pre-ordering with Team Medical is a smart choice?
With only so much stock available, it has never been more important to place your 2026 flu vaccine order early. Acting now ensures you:
• Lock in your supply ahead of the rush.
• Access early bird pricing — maximise value while securing stock.
• Plan with confidence knowing your patients will be protected.
We have fulfilled 100% of 2025 pre-season orders.
Secure your stock with the team that delivers.

CREATE A WORLD FULL OF GIVING WITH US
At Team Medical, every action counts towards making a difference. When you purchase your influenza vaccines from us in 2025, you’re not just protecting your patients – you’re also supporting meaningful causes worldwide.
For every dose of vaccine purchased, we’re committed to providing one day of clean water through projects like borehole construction in Africa. This initiative is part of our partnership with B1G1 – Business for Good, ensuring that our efforts transcend profits to focus on people and purpose.

Our mission goes beyond business success – it’s about creating sustainable lifestyles and making a real difference in communities, both near and far. By choosing Team Medical, you’re contributing to local vaccination efforts and empowering communities overseas with access to clean water.
Together, let’s build a future where every action counts towards a world full of giving.
Correct Storage and Transport is Paramount.
Because, even one broken link in the chain can mean your vaccines aren't viable.
At Team Medical, we adhere to the 'Strive for 5' National Vaccine Storage Guidelines. Below is a snapshot of what we do to ensure your vaccines
are kept at the correct temperature from the moment we receive the stock to when the stock is delivered.

Complete
Coolroom Processing
By picking and packing every order inside our purpose-built coolrooms, we ensure the required temperature range (between 2-8°C) is continually maintained.
Heat & Freeze Monitors
in Every Order
An alarm is triggered if your vaccines get too hot or too cold. For vaccine storage and transport compliance, a freeze monitor is a non-negotiable. Vaccines are damaged once frozen, always check your Heat & Freeze Monitor.

Shipped in Eskies
or in Validated Cold Trucks
In available regions, we ship vaccine orders with Mediport's validated refrigeration vehicles. All other vaccine orders are shipped in eskies following strict procedures.

24-Hour Monitoring
and Backup Power
Our coolrooms are monitored 24 hours a day using back-to-base data loggers. We have generators on all sites to provide backup power when power outages occur.

Door-to-Door
Delivery Tracking
Our dedicated staff track, trace and verify every cold chain delivery, every day. Consignments are checked to ensure arrival at key destination depots.

Complete
Coolroom Processing
By picking and packing every order inside our purpose-built coolrooms, we ensure the required temperature range (between 2-8°C) is continually maintained.
With Team Medical your vaccines arrive in peak condition. We maintain the highest standards and follow strict processes. For your peace of mind and for the integrity
of our business.